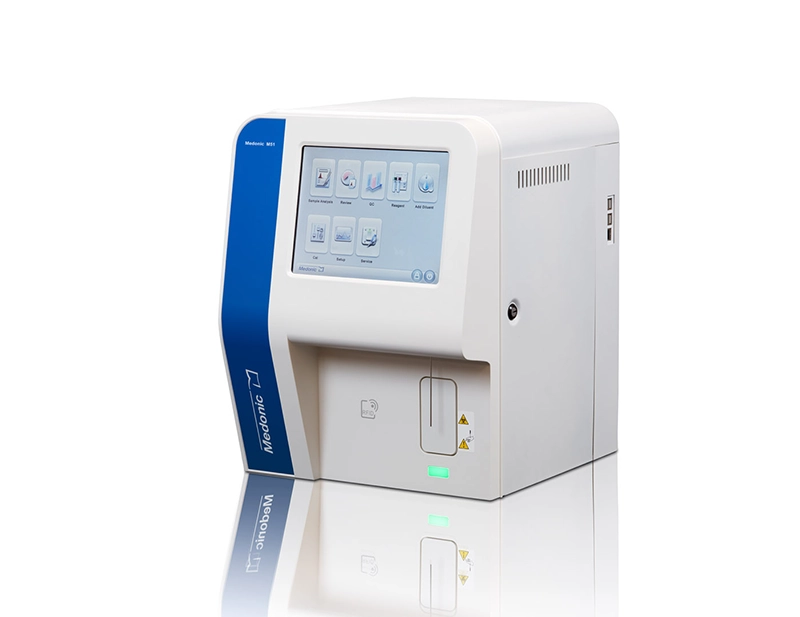
3Part Hematology Analyzer
3Part Hematology Analyzer
Model: M32M (M-Series)
Packed with innovation, Uncompromising accuracy, & Outstanding quality, Melonic M-series M32 analyzer occupy pride of place in the very best hematology labs. There’s room for one on your bench top.
Characteristics
- Sampling to full CBC in about one minute
- Built-in tube mixer
- Micro pipette adapter (MPA)
- Maintenance-free shear valve
- Pre-dilution mode
Specifications
- System: 3Part (Lymphocytes, Monocytes, & Neutrophils)
- Parameters: 22
- Throughput: ≥ 60 samples/hour
- Time to result, OT inlet: ≤ 50 seconds
- MPA: Micro Pipette Adapter option for direct sampling
- Sampling system: Closed shear valve
- Display: 7-inch WVGA true color (24-bit) touch screen
- Interface ports: 1 USB device/4 USB host/1 LAN port
- Memory capacity: 50,000 samples
- QC software: Yes, including Levey-Jennings
Reagents for Medonic M-Series (3Part)
Both RFID & Non-RFID Medonic M-Series uses two (Diluent & Lyse) reagents for analysis. There are two types of reagents packs:
- RFID Locked: these reagents are used in instruments having RFID chips and the tags will scan in order to make it ready for CBC tests. All new instruments are based on this technology.
- Barcoded: these reagents are used in instruments in which RFID chips are not built-in and the barcode will be scanned in order to generate CBC test results. This technology exists in older versions of the instruments.
Cleaning Kit for Medonic M-Series (3Part) Medonic M-Series comes with cleaning kits to perform cleaning of the instruments in order to avoid any kind of relevant problems to the instrument.
Quality Controls for Medonic M-Series (3Part)
The Controls products are provided as part of Total Quality Concept to ensure performance of the analyzer.
- Every day as a routine QC procedure (frequency should reflect workload).
- When the analyzer is restarted after a complete shut-down.
- When changing reagents between lots or within the same manufacturing lot.
- When an unexpected abnormal patient result is reported.

Nawi Hewad Co Ltd is a privately owned Limited Liability Company by shares registered and operating under the laws of Ministry of Industry & Commerce Afghanistan since its foundation in the year 2006.
Get In Touch
- House # 16, 4th Street, Lab-e-Jar Square, Khair Khana III, 1008 Kabul, Afghanistan.
- @Nawihewadcoltd
Copyright – 2024 – Nawi Hewad Co Ltd – All rights reserved. Powered by Msoft Technologies.